Chapter 16
Properties of High-Temperature Gases
Properties of High-Temperature Gases
Overview
As the title indicates, this chapter is devoted to gases at elevated temperatures. The temperature dependence of thermodynamic properties is explained using models for estimation of the inherent energy of molecules (or atoms in the case of mono-atomic gases), i.e. in this chapter we step down from the continuum levels of observation where we study fluid particles to study individual molecules and atoms.
Chapter Roadmap
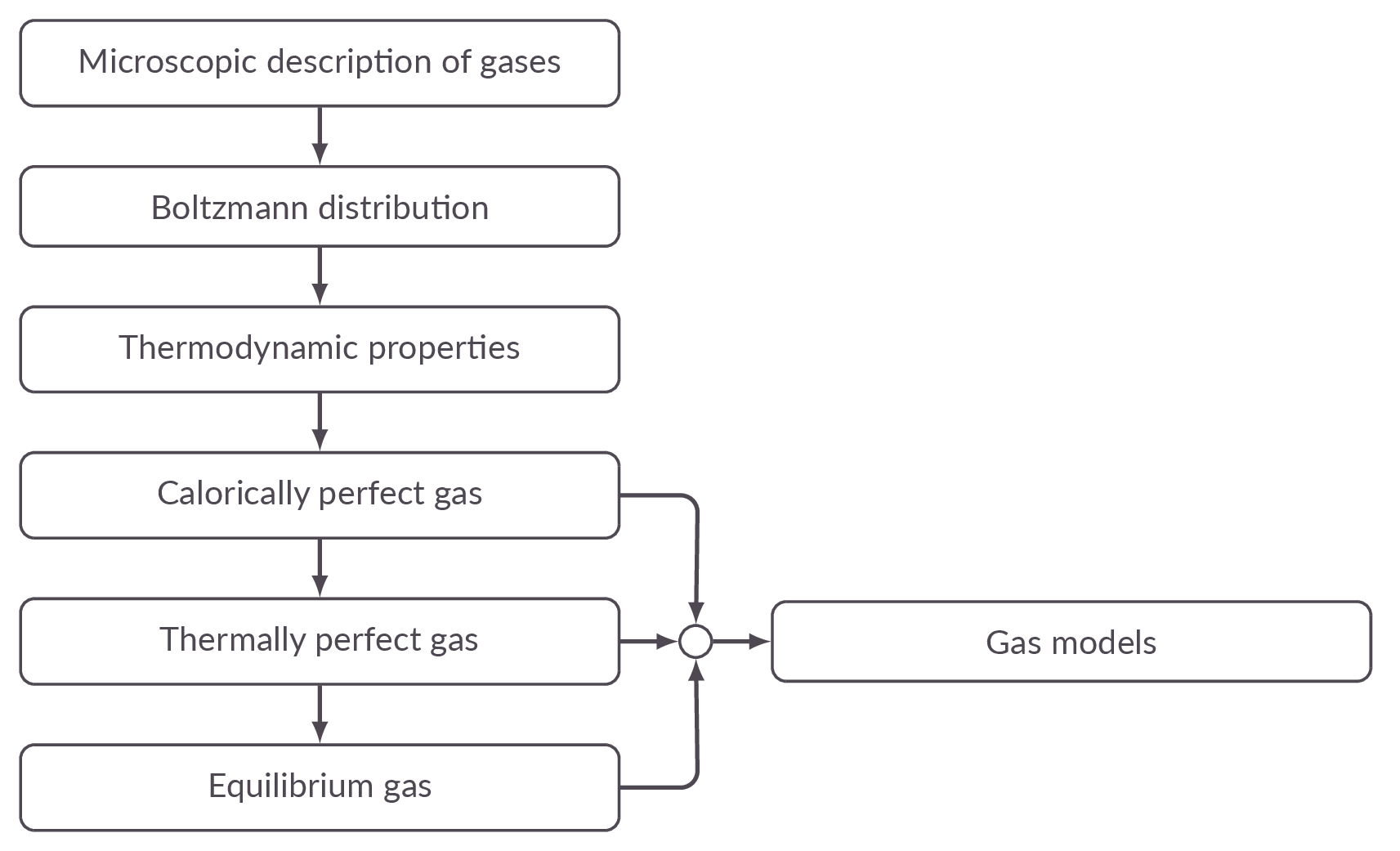
Sections
16.1 Introduction
At high temperature and/or Mach the calorically perfect gas model does not hold and therefore better models are needed. The introduction section gives a few examples that where the calorically perfect gas assumption is used at conditions where it is not applicable in order to highlight the importance of using proper gas models.
16.2 Microscopic Description of Gases
The fundamental energy types of molecules are described; translational energy, rotational energy, vibrational energy, and electronic energy. Each of these energy types is quantized, which means that it can exist in certain discrete values. The lowest possible energy of each type is defined as the zero-point energy. The zero-point energy or the ground state is the energy at zero Kelvin. The rotational zero-point energy is exactly zero whereas the other energy types has finite zero-level values. The electronic energy has the larges zero-point value of the defined energy types. This is explained by the fact that the electrons would fall into the nucleus and the atoms would collapse.
Each possible energy level can occur in different states. It turns out that the energy states are quantized and only occurs in distinguishable discrete values. These are called the degenerate states.
The concept macrostate is introduced and explained and the relation between the most probable macrostate and the population of degenerate states is explained.
16.3 Counting the Number of Microstates for a given Macrostate
This section discusses micro- and macrostates and gives an introduction to the following section.
16.4 The Most Probable Macrostate
The most probable macrostate is the macrostate with the largest number of microstates.
16.5 The Limiting Case: Boltzmann Distribution
The Boltzmann distribution:
$$N_j^*=N\frac{g_j {\mathrm{e}}^{-\varepsilon_j/kT}}{Q}$$where \(Q=f(T,V)\) is the state sum defined as
$$Q\equiv\sum_j g_j {\mathrm{e}}^{-\varepsilon_j/kT}$$\(g_j\) is the number of degenerate states, \(\varepsilon_j=\varepsilon'_j-\varepsilon_o\), and \(k\) is the Boltzmann constant
For molecules or atoms of a given species, quantum mechanics says that a set of well-defined energy levels \(\varepsilon_j\) exists, over which the molecules or atoms can be distributed at any given instant, and that each energy level has a certain number of energy states, \(g_j\).
For a system of \(N\) molecules or atoms at a given \(T\) and \(V\), \(N_j^*\) are the number of molecules or atoms in each energy level \(\varepsilon_j\) when the system is in thermodynamic equilibrium.
16.6 Evaluation of Thermodynamic Properties in Terms of the Partition Function
The state sum \(Q\) defined in the previous section is related to the internal energy of the fluid.
$$E=NkT^2\left(\frac{\partial \ln Q}{\partial T}\right)_V$$$$e=\frac{E}{M}=\frac{NkT^2}{Nm}\left(\frac{\partial \ln Q}{\partial T}\right)_V=\left\{\frac{k}{m}=R\right\}=RT^2\left(\frac{\partial \ln Q}{\partial T}\right)_V$$
16.7 Evaluation of the Partition Function in Terms of T and V
Expressions for each of the energy types are introduced.
$$\varepsilon'_{trans}=\frac{h^2}{8m}\left(\frac{n_1^2}{a_1^2}+\frac{n_2^2}{a_2^2}+\frac{n_3^2}{a_3^2}\right)$$- \(n_1-n_3\) quantum numbers (1,2,3,...)
- \(a_1-a_3\) linear dimensions that describes the size of the system
- \(h\) Planck's constant
- \(m\) mass of the individual molecule
- \(J\) rotational quantum number (0,1,2,...)
- \(I\) moment of inertia (tabulated for common molecules)
- \(h\) Planck's constant
- \(n\) vibrational quantum number (0,1,2,...)
- \(\nu\) fundamental vibrational frequency (tabulated for common molecules)
- \(h\) Planck's constant
16.8 Practical Evaluation of Thermodynamic Properties for a Single Species
The relations introduced in previous sections are used to obtain the ratio of specific heat for mono-atomic and molecule-based gases. For calorically perfect gases, vibrational energy is unpopulated and this results in constant values of \(C_p\) and \(C_v\). When vibrational energy gets populated we switch to thermally perfect gas and analysis of the obtained expressions shows why \(C_p\) and \(C_v\) varies with temperature as temperature is increased.
Translational energy:
$$Q_{trans}=\left(\frac{2\pi mkT}{h^2}\right)^{3/2}V$$$$\ln Q_{trans}=\frac{3}{2}\ln T +\frac{3}{2}\ln\frac{2\pi mk}{h^2}+\ln V$$
$$\left(\frac{\partial \ln Q_{trans}}{\partial T}\right)_V=\frac{3}{2}\frac{1}{T}$$
$$e_{trans}=RT^2\frac{3}{2T}=\frac{3}{2}RT$$
Rotational energy:
$$Q_{rot}=\frac{8\pi^2 IkT}{h^2}$$$$\ln Q_{rot}=\ln T + \ln \frac{8\pi^2 Ik}{h^2}$$
$$\left(\frac{\partial \ln Q_{rot}}{\partial T}\right)_V=\frac{1}{T}$$
$$e_{rot}=RT^2\frac{1}{T}=RT$$
Vibrational energy:
$$Q_{vib}=\frac{1}{1-{\mathrm{e}}^{-h\nu/kT}}$$$$\ln Q_{vib}=-\ln(1-{\mathrm{e}}^{-h\nu/kT})$$
$$\left(\frac{\partial \ln Q_{vib}}{\partial T}\right)_V=\frac{h\nu/kT^2}{\mathrm{e}^{h\nu/kT}-1}$$
$$e_{vib}=\frac{h\nu/kT}{\mathrm{e}^{h\nu/kT}-1}RT$$
$$\lim_{T\rightarrow \infty} \frac{h\nu/kT}{\mathrm{e}^{h\nu/kT}-1}=1\Rightarrow e_{vib}\le RT$$
Internal energy:
$$e=e_{trans}+e_{rot}+e_{vib}+e_{el}$$$$e=\frac{3}{2}RT+RT+\frac{h\nu/kT}{\mathrm{e}^{h\nu/kT-1}}RT+e_{el}$$
Specific heat:
$$C_v\equiv\left(\frac{\partial e}{\partial T}\right)_V$$Molecules with only translational and rotational energy
$$e=\frac{3}{2}RT+RT=\frac{5}{2}RT\Rightarrow C_v=\frac{5}{2}R$$ $$C_p=C_v+R=\frac{7}{2}R$$ $$\gamma=\frac{C_p}{C_v}=\frac{7}{5}=1.4$$Mono-atomic gases with only translational and rotational energy
$$e=\frac{3}{2}RT\Rightarrow C_v=\frac{3}{2}R$$ $$C_p=C_v+R=\frac{5}{2}R$$ $$\gamma=\frac{C_p}{C_v}=\frac{5}{3}=1\frac{2}{3}\simeq 1.67$$Study Guide
The questions below are intended as a "study guide" and may be helpful when reading the text book.
- What are the fundamental modes or forms of energy of a gas molecule?
- How does a mono-atomic gas differ from a diatomic gas in terms of energy modes?
- What is the difference between a linear polyatomic molecule and a nonlinear polyatomic molecule in terms of degrees of thermal freedom?
- What does it mean that the energy is quantized?
- Explain the concept zero-point energy.
- Explain the concept energy state.
- What does macrostate and microstate mean, respectively?
- Try to explain what the Boltzmann distribution describes and what sparsely populated implies.
- Show that the ratio of specific heats (\(\gamma\)) is 1.4 for a molecule with only translational and rotational energy. What will the ratio of specific heats be for a monoatomic gas under the same conditions (explain why)?
- What is the difference between a calorically perfect gas and a thermally perfect gas?
- In what temperature range (approximately) can a gas be assumed to be calorically perfect?
- What happens with the molecules in air at approximately 2500K, 4000K, and 9000K?
- A mixture of chemically reacting perfect gases, where the reactions are always in equilibrium, may be thermodynamically described as a single-species gas. How does this thermodynamic description differ from that of a calorically perfect or thermally perfect gas?
- What is meant by an equilibrium gas?
- Figure 16.11 on page 629 shows how \(C_v/R\) for a diatomic gas develops with temperature. Try to explain the development based on the theory covered in section 16.8. What would the figure look like for a mono-atomic gas?